Production line backgroundXinhanze
With the global promotion of carbon neutrality and sustainable development, the market share of natural graphite anode will increase due to its abundant reserves, low energy consumption and non-toxicity. However, with the development of the industry, people have an increasing demand for improving the long-cycle performance, fast charging capacity, high energy density and thermal stability of natural graphite anodes.
Although natural graphite has significant advantages as a negative electrode material in lithium-ion batteries, there are still many problems that limit the cycle life of natural graphite negative electrodes. One of the most important problems is the repeated cracking and formation of the SEI layer. During the initial lithiation/delithiation process, the decomposition of the electrolyte leads to the formation of a protective SEI layer on the surface of the natural graphite. SEI is not only an excellent conductor of Li +, but also separates the electrolyte from the graphite negative electrode, effectively preventing further decomposition of the electrolyte. However, the SEI on the surface of natural graphite does not have sufficient stability, and the mechanical strength is poor. During long-term operation of lithium batteries, the volume expansion (-9%) due to repeated intercalation/deintercalation of Li between graphite layers can cause SEI rupture. The newly exposed graphite surface further causes the electrolyte to decompose, consuming large amounts of active Li +, resulting in continued reduction of the electrolyte. Another important factor limiting the cycle life of natural graphite anodes is the co-intercalation behavior of solvent molecules. This solvent molecule co-intercalation behavior causes the graphene layer to exfoliate, damaging the graphite structure, thereby rapidly reducing the battery capacity. In addition, the defect sites on the surface of natural graphite can act as active centers, inducing violent electrolyte decomposition, resulting in low coulombic efficiency. In addition, the internal pore defects of natural graphite will also affect its cycle performance. These problems together contribute to the poor cycling performance of natural graphite.
At high temperature, lithium will gradually come out from the graphite, and the highly active lithium on the surface of the graphite will react with the SEI, binder and other substances on the surface of the graphite, which brings huge security risks. The thermal stability can be improved by electrolyte modification and graphite surface modification. In addition, regulating the bulk structure of graphite from the perspective of particle size and defects is also an effective way to improve the thermal stability.
Although natural graphite has significant advantages as a negative electrode material in lithium-ion batteries, there are still many problems that limit the cycle life of natural graphite negative electrodes. One of the most important problems is the repeated cracking and formation of the SEI layer. During the initial lithiation/delithiation process, the decomposition of the electrolyte leads to the formation of a protective SEI layer on the surface of the natural graphite. SEI is not only an excellent conductor of Li +, but also separates the electrolyte from the graphite negative electrode, effectively preventing further decomposition of the electrolyte. However, the SEI on the surface of natural graphite does not have sufficient stability, and the mechanical strength is poor. During long-term operation of lithium batteries, the volume expansion (-9%) due to repeated intercalation/deintercalation of Li between graphite layers can cause SEI rupture. The newly exposed graphite surface further causes the electrolyte to decompose, consuming large amounts of active Li +, resulting in continued reduction of the electrolyte. Another important factor limiting the cycle life of natural graphite anodes is the co-intercalation behavior of solvent molecules. This solvent molecule co-intercalation behavior causes the graphene layer to exfoliate, damaging the graphite structure, thereby rapidly reducing the battery capacity. In addition, the defect sites on the surface of natural graphite can act as active centers, inducing violent electrolyte decomposition, resulting in low coulombic efficiency. In addition, the internal pore defects of natural graphite will also affect its cycle performance. These problems together contribute to the poor cycling performance of natural graphite.
At high temperature, lithium will gradually come out from the graphite, and the highly active lithium on the surface of the graphite will react with the SEI, binder and other substances on the surface of the graphite, which brings huge security risks. The thermal stability can be improved by electrolyte modification and graphite surface modification. In addition, regulating the bulk structure of graphite from the perspective of particle size and defects is also an effective way to improve the thermal stability.
Selected field cases

Carbon nanotube purification production line
Fujian, China
Anode Materials for Sodium Batteries
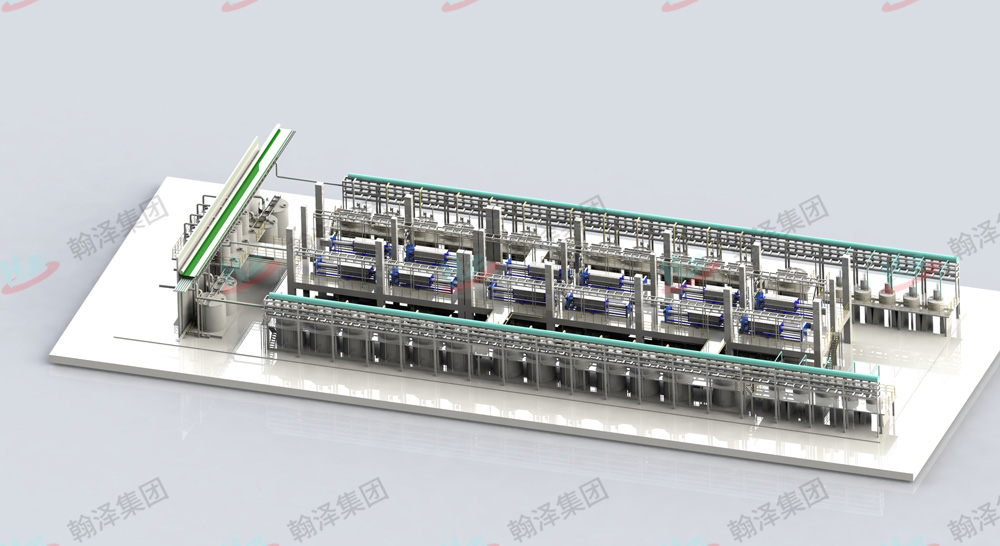
Overseas project site
Indonesia
Unknown

Recycled negative material purification production line
Anhui, China
Recycled negative material

Expandable graphite production line
China
expandable graphite

Natural purification production line
China
natural graphite

Sodium-Hard Carbon Purification Line
Fujian, China
nano battery
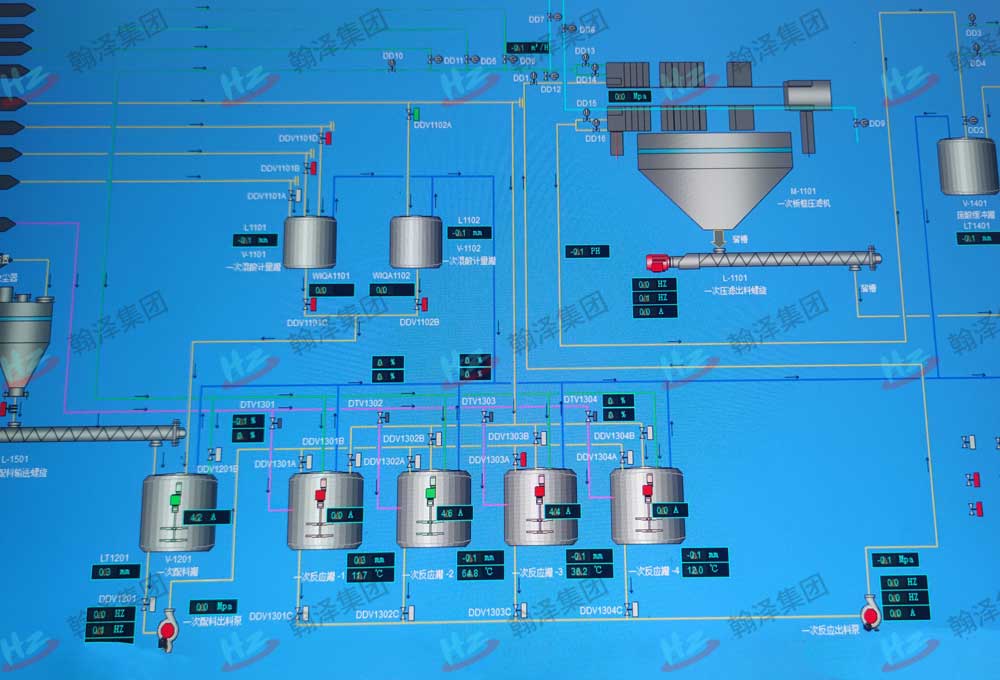
automatic control system
China
Unknown

porous carbonic acid washing production line
China
porous carbonic acid washing

Quartz sand pickling production line
China
Quartz sand pickling

PPH Equipment
China
PP Equipment






